FRAT Test Autism: Complete Guide to Folate Receptor Autoantibody Testing

- TL;DR (Too Long; Didn’t Read)
- What Is the FRAT Test?
- FRAT Test and Autism: The Connection
- Who Should Get the FRAT Test?
- How Is the FRAT Test Done?
- Is the FRAT Test Legitimate?
- Treatment Options for Positive FRAT Test Results
- FRAT Test vs. Other Autism-Related Tests: Comparison Table
- Understanding Cerebral Folate Deficiency (CFD)
- Safety and Side Effects of Folinic Acid Treatment
- Dietary Considerations and FRAT Test
- The Future of FRAT Testing in Autism
- Conclusion
- References and Further Reading (Updated 2026)
TL;DR (Too Long; Didn’t Read)
The Folate Receptor Autoantibody Test (FRAT) is a blood test. It measures autoantibodies directed against folate-receptor alpha (FRα). These autoantibodies can interfere with the transport of folate (vitamin B9) into the brain.
Several studies have reported the presence of folate-receptor autoantibodies (FRAs). Notably, research by Ramaekers, Frye, and Quadros highlights this. They emphasize its significance in a substantial subset of children with autism spectrum disorder (ASD). Reported prevalence varies widely (roughly 40–75% across cohorts). In some studies, children who test positive for these antibodies show measurable improvement in language. They also show enhancement in attention or behavior when treated with high-dose folinic acid (leucovorin).
Crucial Note: The FRAT itself is not a diagnostic test for autism. It may help identify a potentially treatable biological subtype associated with cerebral folate deficiency (CFD).
Are you preparing for an assessment? > Use our Pre-Assessment Clinician Checklist to organize your history. Ask your doctor the right questions about biological markers like the FRAT test.
What Is the FRAT Test?
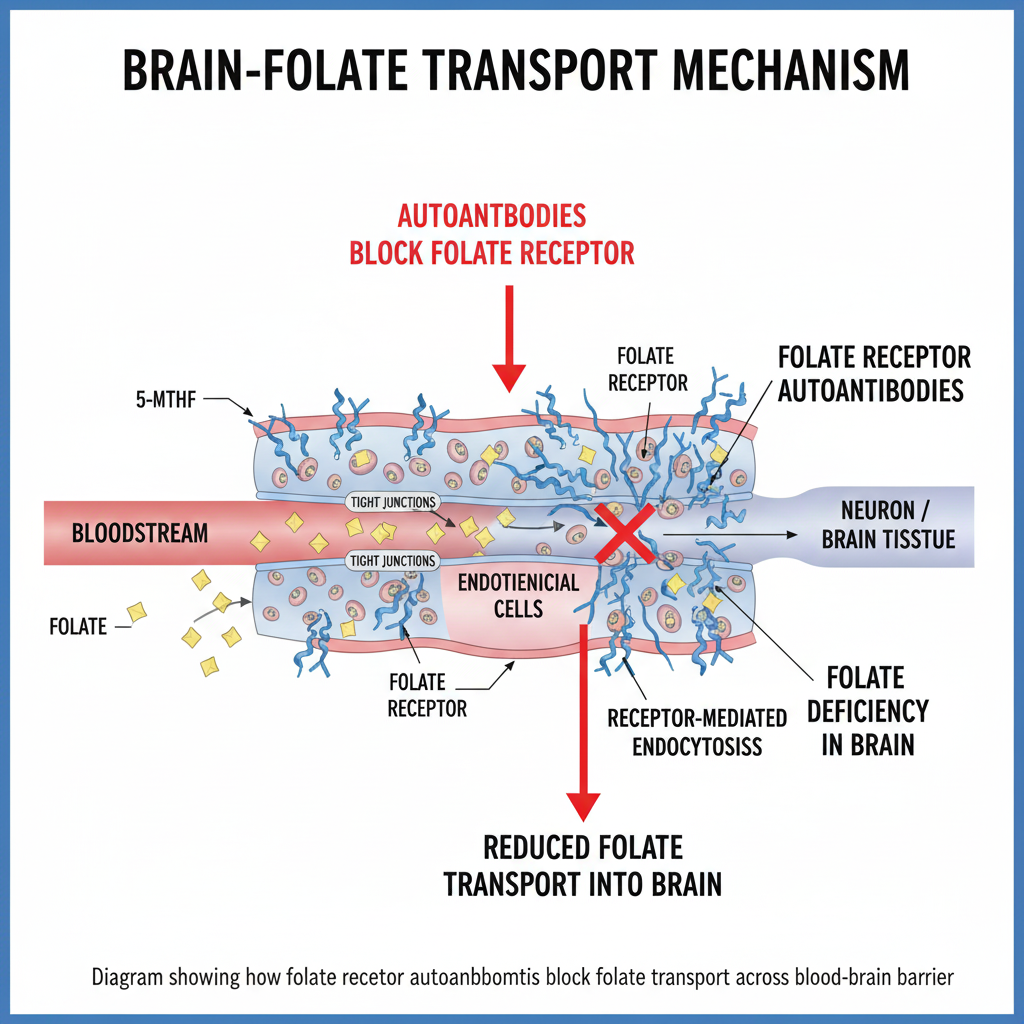
The Folate Receptor Autoantibody Test (FRAT®) was developed at the State University of New York (SUNY Downstate) by Dr. Edward Quadros and colleagues. It is a specialized blood assay that screens for autoantibodies targeting folate receptor alpha (FRα). These antibodies can block the receptor. They may also bind to it. This reduces folate transport across the blood–brain barrier. It potentially lowers folate levels in the central nervous system (CNS).
Understanding Folate Receptor Autoantibodies
Two principal antibody types are detected by the FRAT:
Blocking Autoantibodies (FRAb-B): Prevent folate from binding to its receptor, directly obstructing transport across the blood–brain barrier.
Binding Autoantibodies (FRAb-R): Bind to the receptor. They may alter its conformation or trigger immune responses. These responses impair receptor function even if folate binding still occurs.
When either type is present, reduced folate entry into cerebrospinal fluid (CSF) may occur. This leads to cerebral folate deficiency (CFD). This condition is characterized by low CSF 5-methyltetrahydrofolate (5-MTHF) despite normal blood folate levels.
FRAT Test and Autism: The Connection
Research Findings
In the landmark 2013 Molecular Psychiatry study (Frye et al.), 75% of 93 children with ASD were positive for at least one class of FRAs. Subsequent replication studies have confirmed elevated prevalence but with variable percentages (typically 40–70%), depending on assay methodology and population sampled.
Key observations from peer-reviewed research include:
- Blocking FRAs found in roughly 60% of ASD participants in initial cohorts
- Binding FRAs found in ~45%
- ~30% had both types concurrently
- Children with positive blocking FRAs often exhibit reduced CSF folate concentrations
Importantly, most studies are observational and do not establish a causal link between FRAs and autism itself. The prevailing hypothesis is that, in a biologically susceptible subgroup, folate-transport autoimmunity may contribute to neurodevelopmental symptoms. These symptoms overlap with—or exacerbate—ASD features.
Why This Matters for Autism
Folate is essential for methylation, neurotransmitter synthesis, mitochondrial energy metabolism, and DNA repair. Low CNS folate levels caused by receptor autoantibodies can therefore produce:
- Impaired methylation and gene regulation
- Increased oxidative stress and mitochondrial dysfunction
- Altered neurotransmitter balance and myelination
- Potential contribution to language and attention deficits seen in some ASD children
These processes are biologically testable and, in part, modifiable. As a result, the FRAT may guide targeted metabolic therapy instead of symptom-only management.
Who Should Get the FRAT Test?
The FRAT is most relevant for children or adults with ASD or developmental delays who present with one or more of the following:
- Language regression or persistent speech delay
- Autistic features plus motor or neurological symptoms unexplained by genetics
- Poor response to conventional behavioral or biomedical interventions
- Family history of autoimmune disease
- Low CSF folate documented previously or suspected CFD symptoms
There is evidence suggesting potential clinical benefit across the autism spectrum. However, the test is not routinely ordered outside specialty metabolic or integrative clinics. Consultation with a developmental pediatrician or neuro-immunologist is recommended.
How Is the FRAT Test Done?
Testing Procedure
The FRAT requires a simple peripheral blood draw (≈1 ml serum). The sample is shipped to a laboratory licensed to perform the proprietary FRα antibody ELISA. Processing usually takes 2–4 weeks, after which results are reported as negative, low, moderate, or high titers.
Test Result Categories
Reference ranges may vary slightly by laboratory, but commonly accepted interpretive categories are:
- Blocking FRA: Negative < 0.2 pmol/ml | Low 0.2–0.5 | Moderate 0.5–1.0 | High > 1.0 pmol/ml
- Binding FRA: Negative < 0.5 pmol/ml | Low 0.5–2 | Moderate 2–10 | High > 10 pmol/ml
Even low-positive titers can be clinically meaningful when consistent with symptoms of CFD. Interpretation should always be performed by a clinician familiar with folate metabolism and autoimmune neurology.
Is the FRAT Test Legitimate?
Scientific Validation
The FRAT is based on patented, peer-reviewed methodology developed in academic laboratories and cited in leading journals such as the New England Journal of Medicine (2005) and Molecular Psychiatry (2013). It has been utilized clinically for over a decade. However:
- It is not FDA-cleared or widely available in hospital reference labs.
- It is considered a specialty test with limited commercial distribution (e.g., ReligenDx in the U.S.).
- Insurance coverage is variable and often out-of-pocket.
- Professional societies (e.g., AAP, AACAP) have not yet included FRAT in standard autism evaluation guidelines.
Thus, the test is scientifically credible but remains a research-supported adjunct, not a routine clinical screening tool.
Treatment Options for Positive FRAT Test Results
Folinic Acid (Leucovorin) Therapy
For patients with confirmed FRAs or documented CFD, high-dose folinic acid (leucovorin calcium) can bypass the blocked FRα. It does this by utilizing the reduced-folate carrier (RFC) pathway. Randomized, placebo-controlled trials (Frye et al., 2016 & 2021) have shown statistically significant improvements in verbal communication and adaptive behavior in some FRAT-positive children.
Treatment Protocol
Typical Dosing Range (from clinical studies):
- ≈ 1–2 mg per kg body weight per day (usually capped at 50 mg/day)
- Divided into two daily doses to reduce GI discomfort
- Gradual titration over 1–2 weeks recommended to limit behavioral activation
- Adjunct vitamin B12 and monitoring for B12 status are advised
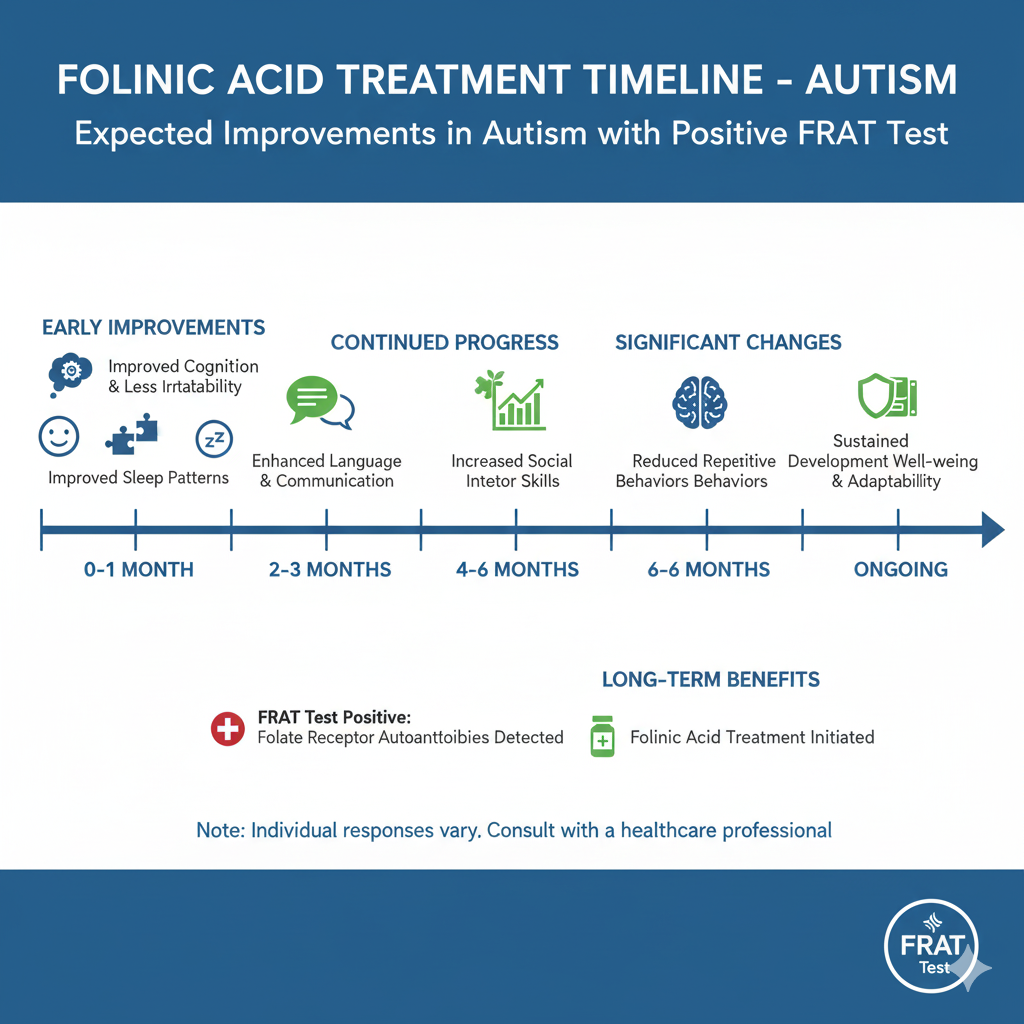
Treatment Duration and Monitoring: Initial review after 8–12 weeks; long-term benefit often evaluated over 6–12 months. Continued therapy is considered safe under medical supervision but should include periodic assessment of behavioral and metabolic parameters.
Expected Improvements
Folinic acid may improve selected behavioral and language domains in certain children with autism. Controlled trials and open-label studies support this conclusion. The most consistent benefits involve language and social-communication gains, though individual response is variable.
- Verbal communication: Enhanced spontaneous speech and responsiveness.
- Receptive and expressive language: Measurable improvements on standardized assessments (e.g., CELF, Vineland).
- Attention and adaptive function: Better focus and social engagement.
- Reduction in stereotypical behaviors: Moderate decrease reported in several cohorts.
Across studies (Frye 2016; Frye et al. 2021 Front Neurosci; Ramaekers 2024 Dev Med Child Neurol), approximately two-thirds of FRA-positive participants show measurable improvement and one-third demonstrate moderate-to-substantial benefit. Younger children and those with blocking-antibody dominance often respond more robustly. Non-responders usually show partial or transient effects rather than adverse outcomes.
Prescription and Availability
Prescription formulations: Pharmaceutical leucovorin calcium (available as 5-, 10-, 25-, and 50-mg tablets) requires a medical prescription. Compounding pharmacies can prepare liquid suspensions for pediatric dosing. Over-the-counter folinic acid supplements exist but are typically lower dose and vary in bioavailability.
Important: Folinic acid (leucovorin) differs chemically from folic acid and from methylfolate. Substitution should be guided by a clinician experienced in metabolic or neuro-immunologic care.
FRAT Test vs. Other Autism-Related Tests: Comparison Table
| Test Name | What It Measures | Type | Primary Purpose | Typical Age Range | Treatment Available |
|---|---|---|---|---|---|
| FRAT Test | Folate-receptor autoantibodies (FRα) | Blood test | Detects possible cerebral folate deficiency subtype | All ages | Yes (folinic acid) |
| M-CHAT-R/F | Screening behaviors | Parent questionnaire | Early autism screening | 16–30 months | N/A |
| ADOS-2 | Observed social and communication behavior | Clinical observation | Diagnostic assessment | 12 months – adult | N/A |
| ADI-R | Developmental and behavioral history | Structured interview | Diagnostic assessment | 2 + years | N/A |
| Genetic Testing | Chromosomal or gene variants | Blood/saliva | Identify genetic etiologies | All ages | Variable |
| MTHFR Genotyping | Folate-metabolism gene variants | Blood/saliva | Assess methylation capacity | All ages | Yes (methylfolate supplementation) |
| Metabolic Panel | Metabolic markers | Blood/urine | Screen for metabolic disorders | All ages | Condition-specific |
| EEG | Brain electrical activity | Electrophysiology | Detect seizures/abnormalities | All ages | Antiepileptic therapy |
Key Distinctions
The FRAT is unique among autism-related evaluations. It directly measures an immune mechanism that impairs nutrient transport. This is different from evaluations that focus on behavior or genetics. It provides actionable biochemical information. This information may guide specific treatment. In contrast, diagnostic or behavioral instruments classify symptoms but do not address etiology.
Expert Guidance by Dror Arbel | Founder of 101Autism.com
Your journey doesn’t end with a score. Take the next step toward a formal diagnosis by using this Pre-Assessment Clinician Checklist to organize your symptoms, gather documentation, and prepare for your appointment.
🖨️ Download the Official Checklist (PDF)Understanding Cerebral Folate Deficiency (CFD)
Cerebral Folate Deficiency (CFD) describes low concentrations of 5-methyltetrahydrofolate in cerebrospinal fluid despite normal blood folate. The most common cause in children is folate-receptor autoimmunity, though mitochondrial, genetic, or pharmacologic causes also occur.
- Primary cause: FRα autoantibodies (~70–80 % of cases)
- Other contributors: mitochondrial disorders, MTHFR or RFC gene variants, antiepileptic drugs (valproate, carbamazepine), and nutritional insufficiency.
CFD can present with speech or motor regression, hypotonia, seizures, or autistic-like features. The “gold standard” diagnostic test remains CSF 5-MTHF measurement by lumbar puncture. However, FRAT offers a validated, non-invasive screening alternative. It has good positive-predictive value.
Safety and Side Effects of Folinic Acid Treatment
Across multiple trials, folinic acid has demonstrated a strong safety profile. Most children tolerate therapy well when doses are titrated gradually.
- Mild gastrointestinal discomfort or loose stools (≤ 10%)
- Sleep or activity changes (5–15%)
- Transient hyperactivity—usually resolves with dose adjustment
- Rare behavioral aggravation when combined with antipsychotics such as risperidone (Frye 2016)
Monitoring includes observation of behavior, growth, and B12 status every few months. No significant biochemical toxicity has been reported in long-term follow-up studies up to 3 years.
Dietary Considerations and FRAT Test
Cow’s-milk proteins have structural homology with FRα epitopes and can provoke or sustain antibody formation in susceptible individuals. Studies (Ramaekers et al., 2008; Blau 2022 Nutrients) showed that a strict milk-free diet may reduce antibody titers and enhance response to folinic acid therapy.
- Eliminate cow’s and goat’s milk and casein-containing foods.
- Prioritize folate-rich plant sources—leafy greens, lentils, asparagus, avocado, citrus.
- Note: dietary folate cannot by itself correct antibody-mediated transport blockage; supplementation remains necessary.
The Future of FRAT Testing in Autism
Current research is exploring precision-medicine models in autism that integrate immune, metabolic, and genetic biomarkers. FRAT represents one of the most reproducible immunologic markers so far identified for a treatable ASD subgroup.
- Ongoing multicenter trials (Frye 2024, NCT06091234) evaluating long-term outcomes.
- Investigation of prenatal FRA exposure and maternal autoimmunity (Sequeira 2023).
- Integration with metabolomic and mitochondrial profiling.
- Studies on combined folinic-acid + B12 or antioxidant therapy.
As evidence grows, professional guidelines may eventually incorporate FRAT screening for children with unexplained developmental regression or metabolic-autistic features.
Conclusion
The Folate Receptor Autoantibody Test offers an evidence-based perspective. It relates to a biologically distinct subset of autism spectrum disorder. This subset is linked to cerebral folate deficiency. While not diagnostic of autism itself, FRAT can identify children who may respond to targeted metabolic therapy with folinic acid. Clinical data up to 2025 confirm its safety. They show moderate efficacy in improving communication. Adaptive behaviors improve when it is used under professional supervision.
Families considering FRAT testing should do so in collaboration with qualified healthcare providers who understand both autism and metabolic-immune interactions. When interpreted correctly, the test offers a personalized and hopeful adjunct to comprehensive autism care.
Expert Resources from Dror Arbel:
- Scoring Tools: SRS-2 Scoring Guide
- Assessment Preparation: Adult Autism Cost Guide
- Recommended Sensory Gear: 2026 Product Guide
References and Further Reading (Updated 2026)
- Frye RE et al. Cerebral folate receptor autoantibodies in autism spectrum disorder. Molecular Psychiatry. 2013; 18:369-381.
- Ramaekers VT et al. Autoantibodies to folate receptors in the cerebral folate deficiency syndrome. New England Journal of Medicine. 2005; 352:1985-1991.
- Frye RE, Slattery J, Quadros EV et al. Folinic acid improves verbal communication in autism spectrum disorder: a randomized controlled trial. Molecular Psychiatry. 2016; 21:241-250.
- Frye RE et al. Mechanistic insights and long-term outcomes of folinic acid treatment in autism. Frontiers in Neuroscience. 2021; 15:679 791.
- Ramaekers VT & Quadros EV. Cerebral folate deficiency and autoimmunity in neurodevelopmental disorders — an update 2024. Developmental Medicine & Child Neurology. 2024; 66(3):341-352.
- Sequeira JM et al. Maternal folate receptor autoantibodies and offspring neurodevelopment: current status and future directions. Nutrients. 2023; 15(6):1391.
- Blau N, Ramaekers VT et al. Dietary interventions in folate receptor autoimmunity. Nutrients. 2022; 14(19):4084.
- National Institutes of Health. Cerebral Folate Deficiency Syndrome overview. NIH Genetic and Rare Diseases Information Center (GARD). Updated 2024.
- ReligenDx Laboratories. FRAT Test Information Portal. https://www.religendx.com (Accessed Oct 2025).
Disclaimer (2026 update): This article is for educational purposes only. It should not be used as a substitute for professional medical advice. Diagnosis and treatment of autism spectrum disorder or cerebral folate deficiency must be conducted under qualified healthcare supervision.
Discover more from Living with Autism
Subscribe to get the latest posts sent to your email.



2 Responses
[…] Official 101Autism Quiz FRAT Test Guide […]
[…] Official 101Autism Quiz FRAT Test Guide […]